MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Find the quantum number of the excited state of electrons in ion which on the transition to the first excited state emits photons of wavelengths . ()
(a)
(b)
(c)
(d)
37.93% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
JEE Main/Advance
IMPORTANT
Where . What is the maximum radial distance of node from nucleus?
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Consider the following six electronic configurations (remaining inner orbits are completely filled) and mark the correct option.
(I)
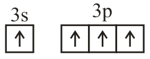
(II)
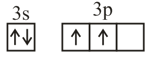
(III)
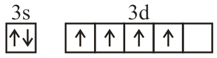
(IV)
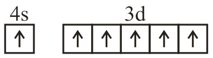
(V)
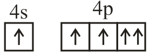
(VI)
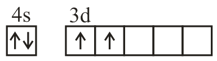
HARD
JEE Main/Advance
IMPORTANT
