EASY
JEE Main
IMPORTANT
Earn 100
Find the ratio of energies of photons produced due to transition of an election of hydrogen atom from its(i) second permitted energy level to the first level, and (ii) the highest permitted energy level to the first permitted level.
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Atoms and Nuclei
HARD
JEE Main
IMPORTANT
Two radioactive materials and have decay constants and respectively. If initially they have the same number of nuclei, then the ratio of the number of nuclei of to that of A will be "" after a time . The value of is _____ .
EASY
JEE Main
IMPORTANT
Consider the following radioactive decay process:
The mass number and the atomic number of are given by:
MEDIUM
JEE Main
IMPORTANT
Assume that protons and neutrons have equal masses. Mass of a nucleon is and radius of nucleus is . The approximate ratio of the nuclear density and water density is . The value of is _____.
EASY
JEE Main
IMPORTANT
A photon is emitted in transition from level in hydrogen atom. The corresponding wavelength for this transition is (given, )
EASY
JEE Main
IMPORTANT
The energy released per fission of nucleus of is . The energy released if all the atoms in of pure undergo fission is _____.
(Given )
EASY
JEE Main
IMPORTANT
The ratio of the density of oxygen nucleus and helium nucleus is
MEDIUM
JEE Main
IMPORTANT
The wavelength of the radiation emitted is when an electron jumps from the second excited state to the first excited state of hydrogen atom. If the electron jumps from the third excited state to the second orbit of the hydrogen atom, the wavelength of the radiation emitted will be . The value of is________.
EASY
JEE Main
IMPORTANT
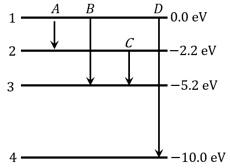
The energy levels of an atom is shown is figure.
Which one of these transitions will result in the emission of a photon of wavelength ?
Given ()