EASY
JEE Main
IMPORTANT
Earn 100
First law of thermodynamics corresponds to
(a)conservation of energy
(b)heat flow from hotter to colder body
(c)law of conservation of angular momentum
(d)Newton's law of cooling
(e)law of conservation of linear momentum
100% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
EASY
JEE Main
IMPORTANT
A system is taken from state to state along two different paths and The heat absorbed and work done by the system along these two paths are and and and , respectively. Then
EASY
JEE Main
IMPORTANT
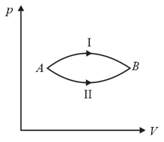
A certain amount of an ideal gas is taken from state to state , one time by the process I and another time by process II. If the amount of heat absorbed by the gas are and respectively, then
EASY
JEE Main
IMPORTANT
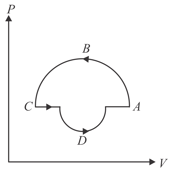
Work done by the gas in the process shown in figure is
EASY
JEE Main
IMPORTANT
Identify the incorrect statement related to a cyclic process.
EASY
JEE Main
IMPORTANT
An ideal gas of volume and at pressure is supplied with of energy. The volume increases to the pressure remaining constant. The internal energy of the gas is
EASY
JEE Main
IMPORTANT
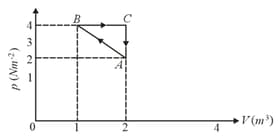
Corresponding to the process shown in the figure, what is the heat given to the gas in the process
EASY
JEE Main
IMPORTANT
A closed system undergoes a change of state by process for which and The system is now returned to its initial state by a different path for which is . The work done by the gas in the process is
EASY
JEE Main
IMPORTANT
A thermodynamic system is changed from state to by two different process. The quantity which will remain same will be?
