HARD
Earn 100
Following Kjeldahl's method, of organic compound released ammonia, that neutralised of The percentage of nitrogen in the compound is _______
50% studentsanswered this correctly
Important Questions on Purification and Characterisation of Organic Compounds
EASY
Which of the following is used for the estimation of halogens in organic compounds?
HARD
The Kjeldahl method of Nitrogen estimation fails for which of the following reaction products?
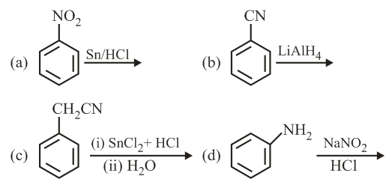
HARD
In the Kjeldahl's method of estimation of nitrogen, ammonia gas evolved from of an organic compound was absorbed in of sulphuric acid. The unused acid required of for complete neutralisation. The weight percentage of nitrogen in the organic compound is
HARD
1.4 g of an organic compound was digested according to Kjeldahl's method and the ammonia evolved was absorbed in 60 mL of M/10 solution. The excess sulphuric acid required 20 mL of M/10 NaOH solution for neutralization. The percentage of nitrogen in the compound is:
EASY
An organic compound ‘A’ is oxidized with followed by boiling with The resultant solution is then treated with ammonium molybdate to yield a yellow percipitate. Based on a above observation, the element present in the given compound is:
EASY
Which of the following is a FALSE statement?
MEDIUM
In Carius method of estimation of halogen, of an organic compound showed presence of of bromine. Which of these is the correct structure of the compound ?
MEDIUM
In Duma's method of estimation of nitrogen, of an organic compound gave of nitrogen collected at and of pressure. The percentage composition of nitrogen in the compound is ______. (Round off to the Nearest Integer).
[Given : Aqueous tension at of ]
MEDIUM
In Duma's method for estimation of nitrogen, of an organic compound gave of nitrogen collected at temperature and pressure. If the aqueous tension at is , the percentage of nitrogen in the compound is:
EASY
In the Kjeldahl's method for estimation of nitrogen present in soil sample, ammonia evolved from of sample neutralized of The percentage of nitrogen in the soil is:
HARD
Complete combustion of of an oxygen containing compound gave of and of The percentage of oxygen in the organic compound is :
MEDIUM
For the estimation of nitrogen, of an organic compound was digested by the Kjeldahl method and the evolved ammonia was absorbed in of sulphuric acid. The unreacted acid required of sodium hydroxide for complete neutralization. The percentage of nitrogen in the compound is
MEDIUM
The formula of a gaseous hydrocarbon which requires 6 times of its own volume of for complete oxidation and produces times its own volume of is The value of is ________.
HARD
An organic compound weighing gave on Carius estimation, of . The percentage of in the compound will be close to At. Mass
EASY
Nitrogen can be estimated by Kjeldahl's method for which of the following compound?
MEDIUM
of an organic compound was analysed by Kjeldahl's method for the estimation of nitrogen. If the percentage of nitrogen in the compound was found to be , then....... of would have been neutralized by the ammonia evolved during the analysis.
EASY
In Carius method, halogen containing organic compound is heated with fuming nitric acid in the presence of:
MEDIUM
Kjeldahl’s method cannot be used to estimate nitrogen for which of the following compounds?
MEDIUM
Which of the following compounds will be suitable for Kjeldahl's method for nitrogen estimation?
EASY
The ammonia evolved from of a compound in Kjeldahl's estimation of nitrogen neutralizes solution. The weight percentage of nitrogen in the compound is:

