MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Following figure shows an unit cell with atoms of radius marked (corner ), (face center), (face center). A quadrilateral is also shown by joining the centers of face centered atoms. Find:
(i) The distances between atoms and .
(ii) The shape and dimensions of the quadrilateral.
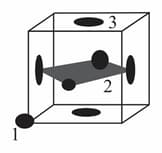
(i) The distances between atoms and .
(ii) The shape and dimensions of the quadrilateral.

Important Questions on Solid State
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Silicon is an insulator, but silicon doped with phosphorus acts as a semiconductor.
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
At room temperature, Polonium crystallises in cubic primitive cell. If edge length is . Calculate the theoretical density of . (Atomic wt of )
