Following figure shows dependence of molar conductance of two electrolytes on concentration. is the limiting molar conductivity.
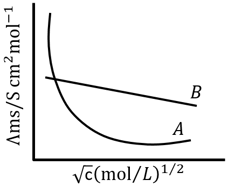
The number of Incorrect statement(s) from the following is _____
(A) for electrolyte is obtained by extrapolation
(B) For electrolyte graph is a straight line with intercept equal to
(C) At infinite dilution, the value of degree of dissociation approach zero for electrolyte .
(D) for any electrolyte or can be calculated using for individual ions.

(B) For electrolyte graph is a straight line with intercept equal to
(C) At infinite dilution, the value of degree of dissociation approach zero for electrolyte .
(D) for any electrolyte or can be calculated using for individual ions.

Important Questions on Electrochemistry
The equilibrium constant for the reaction is at . The magnitude of standard electrode potential of if is _____ . (Nearest integer)
Given :
Consider the cell
When the potential of the cell is at , the ratio is
(Nearest integer)
Given:
The electrode potential of the following half cell at
is _____ (Nearest integer)
Given :
Which one of the following statements is correct for electrolysis of brine solution?
The logarithm of equilibrium constant for the reaction is
(Nearest integer)
Given:
At what , given half cell will have electrode potential of ? (Nearest Integer)
Given
is added to of saturated solution of . The conductivity of this solution at is .
[Given : at
