HARD
Earn 100
Following is the graph between log t1/2 and log a (a = initial concentration) for a given reaction at 27oC. Hence, order is :
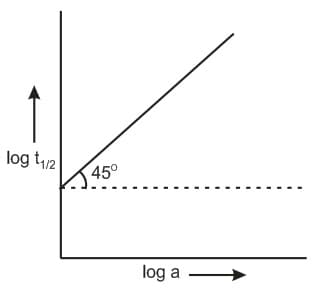
(a)0
(b)1
(c)2
(d)3
45.19% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
EASY
MEDIUM
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
is zero
EASY
EASY

