MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Following represent the Maxwell distribution curve for an ideal gas at two temperature and Which of the following option(s) are true?
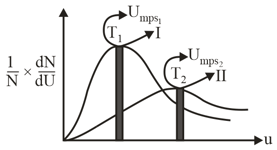

(a)Total area under the two curves is independent of moles of gas.
(b) decreases as temperature decreases.
(c) and hence higher the temperature, sharper the curve.
(d)The fraction of molecules having speed decreases as temperature increases.
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
MEDIUM
JEE Main/Advance
IMPORTANT
If for two gases of molecular weights and at temperature and then which property has the same magnitude for both the gases?
MEDIUM
JEE Main/Advance
IMPORTANT
Gaseous decomposition of follows order kinetics. Pure is taken in a sealed flask where decomposition occurs as
After sec., a leak was developed in the flask. On analysis of the effused gaseous mixture (Obeying Graham's law) coming out initially, moles of were found to be double of . What is rate constant in
Given: Molecular weight of Molecular weight of Molecular weight of
EASY
JEE Main/Advance
IMPORTANT
Viscosity is a measure of resistance of a liquid to flow and viscosity-
MEDIUM
JEE Main/Advance
IMPORTANT
One mole of Ideal gas . follow the process as shown in figure. Predict the following:
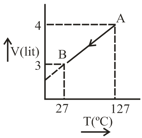
Nature of process
EASY
JEE Main/Advance
IMPORTANT
Internal pressure of a perfect gas (ideal gas) is:
