MEDIUM
Earn 100
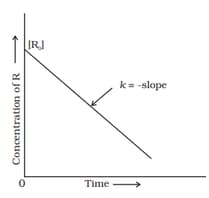
For, a zero-order reaction, the plot of concentration of reactant versus rate is linear with negative slope and zero intercept.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Kinetics (AHL)
MEDIUM
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
HARD
EASY
EASY
HARD
Which of the following relation is correct for zero-order reaction?
EASY
HARD
Examine the graph given below. Identify the integrated rate equation and the order of the reaction corresponding to it.
HARD
MEDIUM
EASY
The given graph is a representation of kinetics of a reaction.
The and axes for zero and first order reactions, respectively are
HARD
Derive the integrated rate equation of Zero Order Reaction.
EASY
MEDIUM
EASY