HARD
Earn 100
For H2S ; , . A Saturated solution of H2S in 0.1 M H2S contains Mn2 + , Co2 + and Ag+ at an original concentration of 0.01 M each. Determine the pH range for selective precipitation of these metal ions
Calculate difference in pH for precipitation of MnS and CoS.
Round off the answer to the nearest integer.
Calculate difference in pH for precipitation of MnS and CoS.
50% studentsanswered this correctly
Important Questions on Ionic Equilibrium
MEDIUM
MEDIUM
HARD
MEDIUM
EASY
MEDIUM
HARD
MEDIUM
HARD
HARD
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
MEDIUM
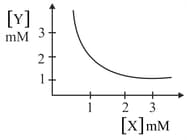
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
EASY
EASY
MEDIUM
HARD
EASY

