MEDIUM
JEE Main
IMPORTANT
Earn 100
For molecule consider the following:
(A) Number of lone pairs on oxygen is .
(B) angle is less than .
(C) Oxidation state of is .
(D) Molecule is bent '' shaped.
(E) Molecular geometry is linear.
Correct options are:
(A) Number of lone pairs on oxygen is .
(B) angle is less than .
(C) Oxidation state of is .
(D) Molecule is bent '' shaped.
(E) Molecular geometry is linear.
(a)C, D, E only
(b)B, E, A only
(c)A, C, D only
(d)A, B, D only
80% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main
IMPORTANT
Match List I with List II
| List I | List II |
| A. | I.See-saw |
| B. | II. Square planar |
| C. | III. Bent shaped |
| D. | IV. Tetrahedral |
Choose the correct answer from the options given below :
HARD
JEE Main
IMPORTANT
Amongst the following, the number of species having the linear shape is
and
MEDIUM
JEE Main
IMPORTANT
Resonance in carbonate ion is
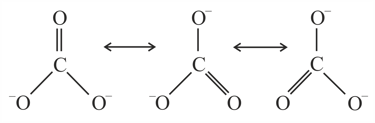
Which of the following is true?
HARD
JEE Main
IMPORTANT
bond length in is than the bond length in . The bond length in is than that of the bond in . Choose the correct option for and from the given below.
