EASY
Earn 100
For a 4p orbital, the number of radial and angular nodes, respectively, are
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Structure of Atom
MEDIUM
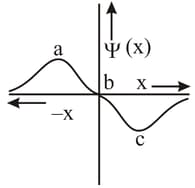
MEDIUM
[Planck's constant ]
EASY
The correct order of their increasing energies will be:
EASY
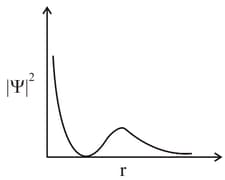
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
An electron in an orbital of high angular momentum stays away from the nucleus than an electron in the orbital of lower angular momentum.
For a given value of the principal quantum number, the size of the orbit is inversely proportional to the azimuthal quantum number.
According to wave mechanics, the ground state angular momentum is equal to .
The plot of Vs for various azimuthal quantum numbers, shows peak shifting towards higher value.
MEDIUM
EASY
EASY
MEDIUM
EASY
HARD
MEDIUM
EASY

