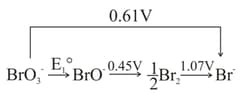EASY
Earn 100
For a cell reaction involving a two electron change, the standard emf of the cell is found to be 0.295 V at The equilibrium constant of the reaction, at will be
(a)10
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
MEDIUM
at is approximately:
EASY
MEDIUM
MEDIUM
MEDIUM
The standard emf of the cell and equilibrium constant of the following reaction of
EASY
EASY
MEDIUM
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
EASY
MEDIUM
is
EASY
MEDIUM
The standard e.m.f. of the cell, in which the cell reaction is is at and at . The enthalpy change of the reaction at is
MEDIUM
MEDIUM
MEDIUM
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
EASY
Given that:
HARD
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and