HARD
JEE Advanced
IMPORTANT
Earn 100
For a certain gas, the heat capacity at constant pressure is greater than that at constant volume by .
(a) How many moles of the gas are there?
(b) If the gas is monatomic, what are heat capacities at constant volume and pressure?
(c) If the gas molecules are diatomic which rotate but do not vibrate, what are heat capacities at constant volume and at constant pressure?

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
EASY
JEE Advanced
IMPORTANT
The heat capacity at constant volume of a sample of a monatomic gas is . Find
(a) the number of moles
(b) the internal energy at
(c) the molar heat capacity at constant pressure.
EASY
JEE Advanced
IMPORTANT
Two thermally insulated vessels and are filled with air at temperatures volumes and pressures , respectively. If the valve joining the two vessels is opened, the temperature inside the vessel at equilibrium will be (common pressure)
EASY
JEE Advanced
IMPORTANT
Two marks on a glass rod apart are found to increase their distance by when the rod is heated from to . A flask made of the same glass as that of rod, measures a volume of at . The volume it measures at in is
EASY
JEE Advanced
IMPORTANT
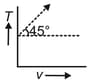
The given curve represents the variation of temperature as a function of volume for one mole of an ideal gas. Which of the following curves best represents the variation of pressure as a function of volume?
EASY
JEE Advanced
IMPORTANT
A gas is found to be obeyed the law constant. The initial temperature and volume are and . If the gas expands to a volume , then the final temperature becomes
EASY
JEE Advanced
IMPORTANT
Air fills a room in winter at and in summer at . If the pressure is the same in winter and summer, the ratio of the weight of the air filled in winter and that in summer is
EASY
JEE Advanced
IMPORTANT
Three closed vessels and are at the same temperature and contain gases which obey Maxwell distribution law of velocities. Vessel contains only and a mixture of equal quantities of and . If the average speed of the molecules in the vessel is that of molecules in vessel is , then the average speed of the molecules in vessel is
EASY
JEE Advanced
IMPORTANT
In a very good vacuum system in the laboratory, the vacuum attained was . If the temperature of the system was , the number of molecules present in a volume of is