EASY
Earn 100
For a chemical reaction, Which of the following is correct?
(a)If then reaction is endothermic
(b)If then reaction is exothermic
(c)If then reaction is endothermic
(d)If then reaction is exothermic
50% studentsanswered this correctly
Important Questions on Equilibrium
EASY
The correct thermodynamic conditions for the spontaneous reaction at all temperatures is:
HARD
The standard state Gibb's free energies of formation of (graphite) and (diamond) at are
The standard state means that the pressure should be 1 bar, and substance should be pure at a given temperature. The conversion of graphite [ (graphite)] to diamond [ (diamond)] reduces its volume by . If (graphite) is converted to (diamond) isothermally at , the pressure at which (graphite) is in equilibrium with (diamond), is
[Useful information: ]
MEDIUM
Using the Gibbs change, for the following reaction, the of in water at is
EASY
For the reaction,
.
What is the value of free energy change, at for the reaction?
HARD
The increase of pressure on system at constant temperature will lead to:
MEDIUM
If for a certain reaction is at the value of (in ) for which the same reaction will be spontaneous at the same temperature is
EASY
A process will be spontaneous at all temperatures if:
MEDIUM
For a reaction and at what temperature reaction changes from spontaneous to non-spontaneous ?
EASY
For a given reaction, and The reaction is spontaneous at : (Assume that and do not vary with temperature)
MEDIUM
For the reaction,
and are, respectively, and at 298 K. The equilibrium constant for the reaction at 298 k is:
MEDIUM
When the heat of a reaction at constant pressure is and entropy change for the reaction is , it is predicted that the reaction at is
HARD
The following reaction is performed at .
The standard free energy of the formation of is at . What is the standard free energy of the formation of at ?
MEDIUM
For the reaction. and of water are and , respectively. Then calculate the value of change in entropy for the given reaction.
MEDIUM
According to the following diagram, A reduces when the temperature is:
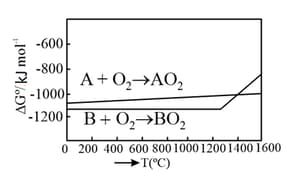
EASY
For a reaction to be spontaneous at all the temperatures, what are the required thermodynamic quantities?
MEDIUM
What is the nature of reaction at , if the entropy change and enthalpy change for a chemical reaction are and, respectively.
MEDIUM
At the temperature for the reaction . Gibbs energy change for the reaction is
Assume are ideal gases
EASY
A reaction at 1 bar is non-spontaneous at low temperature but becomes spontaneous at high temperature. Identify the correct statement about the reaction among the following:
MEDIUM
A process has and Out of the values given below choose the minimum temperature above which the process will be spontaneous:
EASY
The incorrect match in the following is:

