HARD
Earn 100
For a chemical reaction , the mechanism of the reaction postulated was as follows:
If the reaction occurred with individual rate constants and, determine activation energy for the overall reaction if the activation energies associated with these rate constants are and .
(a)
(b)
(c)
(d)
28.57% studentsanswered this correctly
Important Questions on Chemical Kinetics
HARD
EASY
HARD
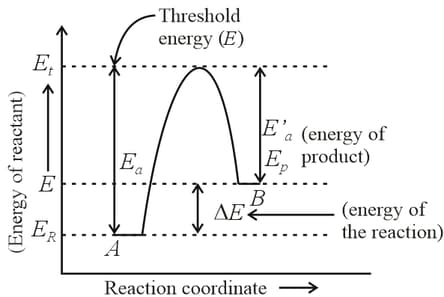
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
EASY
EASY
EASY
MEDIUM
MEDIUM
The reaction rate for the reaction
was measured as a function of concentrations of different species. It was observed that
where square brackets are used to denote molar concentrations. The equilibrium constant
(Nearest integer)
Value of (question is modified.)
MEDIUM
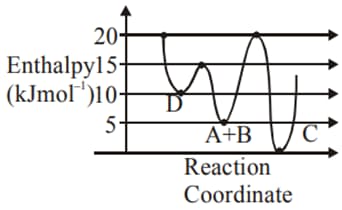
Identify the incorrect statement.
HARD
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
EASY
EASY
MEDIUM
The reaction is an elementary reaction.
For a certain quantity of reactants, if the volume of the reaction vessel is reduced by a factor of the rate of the reaction increases by a factor of _____ . (Round off to the Nearest Integer).
HARD
MEDIUM
MEDIUM
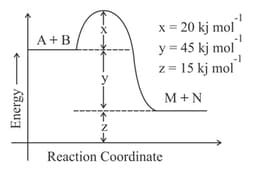
The energy of activation in is ________ . (Nearest integer)
[Given : ]
MEDIUM
in
is equal to __________ . (Integer answer)
EASY
EASY
EASY
(i)
(ii)
In the reaction,

