MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
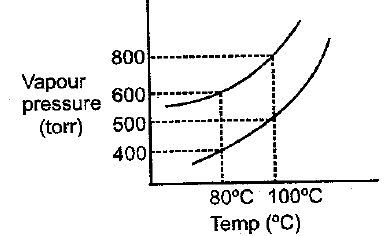
For a dilute solution having molality of a given solute in a solvent of molecular weight , boiling point and heat of vaporisation per mole is equal to:
(a)molal elevation constant of solvent.
(b): where in and in .
(c); where in and in .
(d); where in expressed in same unit of heat.
50% studentsanswered this correctly

Important Questions on Solutions
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
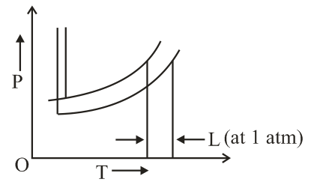
The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by in the figure is:
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
Three different ideal solutions (I, II, III) each containing total moles of in different composition are taken as shown in figure and pressure over the solutions is gradually reduced.
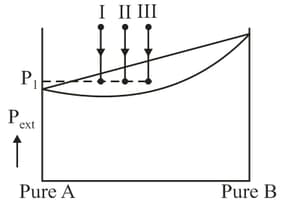
Initially external pressure is same for all three solutions. At a particular external pressure , ll solution is found to have
Then select correct statement:
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
The vapour pressure of ideal solution of benzene and toluene is at then what would be correct statement about same solution at