MEDIUM
Earn 100
For a first order reaction, if the time taken for completion of 50% of the reaction is 't' second, the time required for completion of 99.9% of the reaction is n 't'. Find the value of n?
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
EASY
EASY
EASY
HARD
MEDIUM
MEDIUM
EASY
EASY
HARD
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
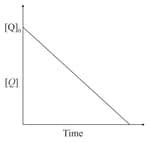
EASY
EASY
HARD
What will be the order of the reaction for hydrolysis of methyl acetate with by using the data provided?
Time
Volume of acid
MEDIUM
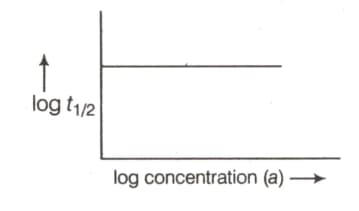
order of reaction
EASY
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
MEDIUM
EASY

