HARD
JEE Advanced
IMPORTANT
Earn 100
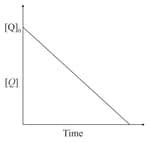
For a first order reaction at constant volume and 300 K, the total pressure at the beginning and at time are , respectively. Initially, only A is present with concentration , and is the time required for the partial pressure of A to reach of its initial value. The correct option(s) is (are)
(Assume that all these gases behave as ideal gases)
(a)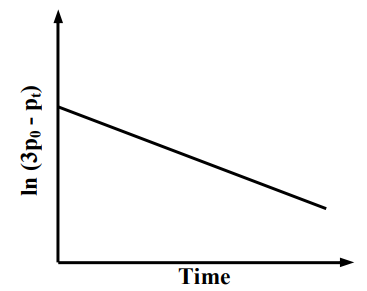

(b)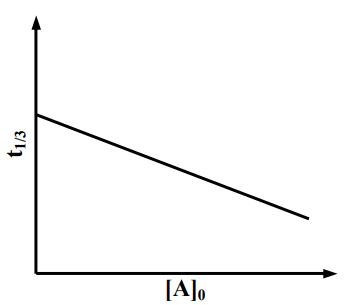

(c)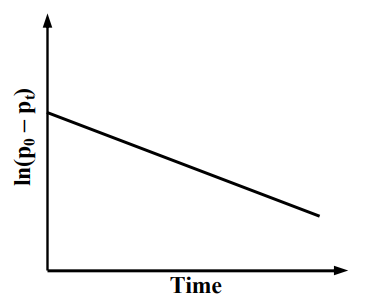

(d)

50% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Advanced
IMPORTANT
Consider the following reversible reaction:
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
HARD
JEE Advanced
IMPORTANT
In a bimolecular reaction, the steric factor was experimentally determined to be . The correct option(s) among the following is(are)
EASY
JEE Advanced
IMPORTANT
For the elementary reaction , the rate of disappearance of increases by a factor of upon doubling the concentration of . The order of the reaction with respect to is
HARD
JEE Advanced
IMPORTANT
is known to undergo radioactive decay to form by emitting alpha and beta particles. A rock initially contained of . If the number of alpha particles that it would emit during its radioactive decay of to in three half-lives is , then what is the value of ?
HARD
JEE Advanced
IMPORTANT
Consider the kinetic data given in the following table for the reaction Product.
| Experiment No. | Rate of reaction | |||
The rate of the reaction for and is found to be The value of is __________
HARD
JEE Advanced
IMPORTANT
According to the Arrhenius equation,
HARD
JEE Advanced
IMPORTANT
In the reaction,
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is: