MEDIUM
Earn 100
For a gas having molar mass of and density of at pressure and temperature. Find the correct option.
(a)Force of attraction is dominating than force of repulsion among the gas molecules.
(b)Force of repulsion is dominating than force of attraction among the gas molecules.
(c)Gas molecules are behaving ideally.
(d)Nothing can be said with certainty.
50% studentsanswered this correctly
Important Questions on States of Matter: Gases and Liquids
MEDIUM
MEDIUM
MEDIUM
( is universal gas constant and is the acceleration due to gravity)
MEDIUM
EASY
EASY
MEDIUM
The volume of gas is twice than that of gas . The compressibility factor of gas is thrice than that of gas at same temperature. What are the pressures of the gases for equal number of moles?
HARD
HARD
MEDIUM
HARD
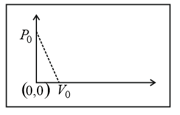
EASY
EASY
EASY
EASY
MEDIUM
HARD
A long cylindrical pipe of radius is closed at its upper end and has an airtight piston of negligible mass as shown. When mass is attached to the other end of piston, it moves down by a distance, before coming to equilibrium. Assuming air to be an ideal gas, (see figure) is close to , one atmospheric pressure is ),
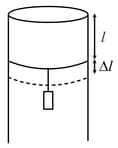
MEDIUM
MEDIUM
The variation of the compressibility factorwith pressure for some gases, are shown in the figure below. Identify the gases and .
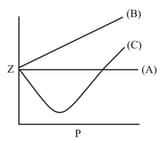
MEDIUM

