For a gas with number of molecules per unit volume, Suppose the molecules of a gas are spheres of diameter the average speed of each molecule is . The time between two successive collisions is on the average
Important Questions on Kinetic Theory
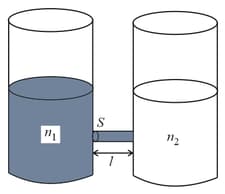
As shown schematically in the figure, two vessels contain water solutions (at temperature ) of potassium permanganate of different concentrations and molecules per unit volume with When they are connected by a tube of small length and cross-sectional area starts to diffuse from the left to the right vessel through the tube. Consider the collection of molecules to behave as dilute ideal gases and the difference in their partial pressure in the two vessels causing the diffusion. The speed of the molecules is limited by the viscous force on each molecule, where is a constant. Neglecting all terms of the order . Which of the following is/are correct? ( is the Boltzmann constant)
An ideal gas in a closed container is slowly heated. As its temperature increases, which of the following statements are true ?
(A) the mean free path of the molecules decreases
(B) the mean collision time between the molecules decreases.
(C) the mean free path remains unchanged.
(D) the mean collision time remains unchanged.
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
Match Column - I and Column - II and choose the correct match from the given choices.
| Column - I | Column - II |
| (A) Root mean square speed of gas molecules | |
| (B) Pressure exerted by ideal gas | |
| (C) Average kinetic energy of a molecule | |
| (D) Total internal energy of of a diatomic gas |

