HARD
NEET
IMPORTANT
Earn 100
For a gaseous phase reaction , the following rate data was obtained at
Rate of disappearance of (mole/litre min)
Concentration
(i)
(ii)
(iii)
The rate constant for the reaction is
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
| Time (sec) | 0 | 10 | 20 | 30 |
| Rate |
From the above data the order of reaction is
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
The rate constant of the production of $2 B(g)$ by the reaction, is
A molar ratio of to in the reaction mixture is attained after
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
If
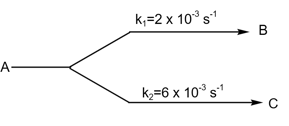
find
