For a gaseous reaction, products, the energy of activation was found to be at . The ratio of the rate constant (k) to the frequency factor (A) at is

Important Questions on Chemical Kinetics
The kinetic data recorded at for the reaction
is
| Set No. | Rate of reaction/ Ms | ||
| 1. | |||
| 2. | |||
| 3. |
The kinetic rate expression and the unit of rate constant (k) of the above reaction are respectively
For an elementary rearrangement reaction , the following data were recorded at when
| Set No. | ||
| 1 | ||
| 2 | ||
| 3 |
If the equilibrium constant of the reaction is 1.12 at , the rate constant for the reaction is:
For the reaction the rate expression is
The correct statement is.
. The reaction is not elementary.
. The reaction is of second order.
. .
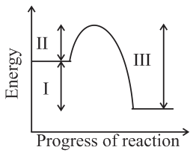
Which of the energy values marked as and in the following diagram, will change by the addition of a suitable catalyst?
Urea, decomposes at as Experimental data obtained for the reaction is given in the following plot;
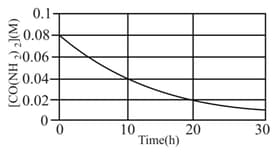
From the graph it can be inferred that-
A simple mechanism for enzyme-catalyzed reaction is given by the following set of equations:
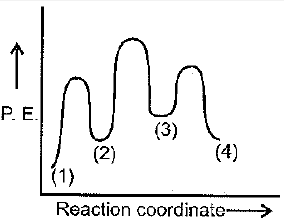
This is known as the Michaelis-Menten mechanism. The potential energy diagram is shown in the figure. Which of the following sets of identifications is correct? (Assume that the temperature and pressure are constant).
