HARD
JEE Main/Advance
IMPORTANT
Earn 100
For a gaseous reaction products, the half-life of the first order decomposition at is minutes and the energy of activation is What fraction of molecules of at have sufficient energy to give the products?

Important Questions on Chemical Kinetics
HARD
JEE Main/Advance
IMPORTANT
At some temperature, the rate constant for the decomposition of on a gold surface is
What is the order of the reaction? How long will it take for the concentration of to drop from .
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
For the complex,
Hence, ratio of rate constants of the forward and backward reaction is:
MEDIUM
JEE Main/Advance
IMPORTANT
Consider the elementary reaction sequence shown in figure. Which of the following equation correct?
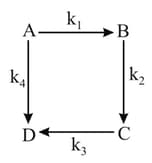
HARD
JEE Main/Advance
IMPORTANT
is a first order reaction,
| Time | ||
| Moles of reagent |
Reaction progress is measure with the help of titration of reagent If all and reacted with reagent and have factors [ factors; eq.wt ] in the ratio of with the reagent. The in terms of is:
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
The energy of activation for the three steps are , and respectively. Therefore:
