MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
For a hypothetical elementary reaction.
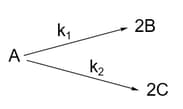
where
Initially, only moles of are present. The total number of moles of and at the end of of reaction are:

(a)
(b)
(c)
(d)
35% studentsanswered this correctly

Important Questions on Chemical Kinetics
HARD
JEE Main/Advance
IMPORTANT
For the following parallel chain reaction 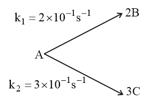 , if the sum of the concentration of and at any time is . What will be and respectively?
, if the sum of the concentration of and at any time is . What will be and respectively?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
The reaction of and is first order in and
The reaction can take place by mechanism:
Select correct mechanism.
MEDIUM
JEE Main/Advance
IMPORTANT
The reaction of hydrogen and iodine monochloride is represented by the equation:
This reaction is first-order in and also first-order in . Which of these proposed mechanisms can be consistent with the given information about this reaction?
Mechanism :
Mechanism :
