HARD
JEE Main/Advance
IMPORTANT
Earn 100
For a monoatomic gas, the value of the ratio of and is:
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
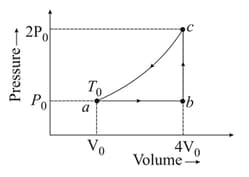
One mole of an ideal monoatomic gas is made to go through the cycle as shown in figure. Then the change in the internal energy in expanding the gas from to along the path is
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
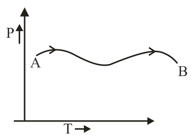
The graph as given below was observed for a process on an ideal gas, which of the following statement is true.
EASY
JEE Main/Advance
IMPORTANT
The difference between heats of reaction at constant pressure and constant volume for the reaction
at in is:-.
