HARD
12th West Bengal Board
IMPORTANT
Earn 100
For a reaction,
When concentration of is doubled, the rate of reaction becomes two-times of the original. When the concentration of is doubled the rate becomes four times. What is the order of the reaction ?
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
12th West Bengal Board
IMPORTANT
For the reaction
The value of would be:
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
EASY
12th West Bengal Board
IMPORTANT
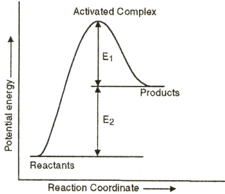
EASY
12th West Bengal Board
IMPORTANT
Consider a first-order gas-phase decomposition reaction given below:
The initial pressure of the system before decomposition of was After the lapse of time total pressure of the system increased by units and became The rate constant for the reaction is given as-
