MEDIUM
JEE Main
IMPORTANT
Earn 100
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
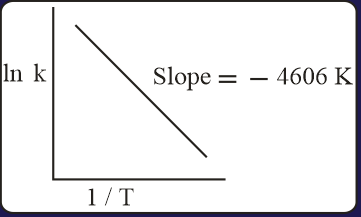

(a)
(b)
(c)
(d)
85.71% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
JEE Main
IMPORTANT
Decomposition of exhibits a rate constant of . How many years are required for the decomposition of of into ?
EASY
JEE Main
IMPORTANT
required for a reaction is produced by the decomposition of in as per the equation,
The initial concentration of is and it is after 30 minutes. The rate of formation of is:
MEDIUM
JEE Main
IMPORTANT
In the following reaction;
‘A’ and ‘B’ respectively can be:
EASY
JEE Main
IMPORTANT
, the rate equation for the reaction is given as
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
EASY
JEE Main
IMPORTANT
For the equilibrium, . If the ratio of the activation energies of the forward and reverse reactions is then:
HARD
JEE Main
IMPORTANT
For an elementary chemical reaction, , the expression for is:
HARD
JEE Main
IMPORTANT
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
JEE Main
IMPORTANT
For the reaction of with , the rate constant is at and at . The activation energy for the reaction, in is:
