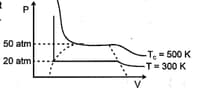
For a real gas, the curve was experimentally plotted, and it had the following appearance. With respect to liquefaction, choose the correct statement:


Important Questions on States of Matter: Gases and Liquids
Consider the following statements:
The coefficient B in the Virial equation of state
(i) is independent of temperature
(ii) is equal to zero at Boyle temperature
(iii) has the dimension of molar volume, Which of the above statements are correct.
Which of the above statements are correct.
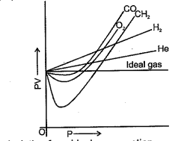
The curve of pressure volume against pressure of the gas at a particular temperature is as shown, according to the graph which of the following is incorrect (in the low pressure region):
mole of vapours at occupies a volume of .If Van der Waal's constant are and , calculate compressibility factor under,
low pressure region high pressure region
Report your answer as nearest whole number of .
To an evacuated steel container, is added and the temperature is raised to causing a complete decomposition of the salt. If the density of formed is find the accurate pressure developed in the container using the Van der Waals equation of state. The Van der Waals constants for are
Given that atomic weight of .
Report your answer as nearest whole number.
