HARD
JEE Main/Advance
IMPORTANT
Earn 100
For a second order reaction plots are made for vs time for the reaction, Product. Pick up the correct sentences. Rate constant of
(a)the graph will show straight line with slope
(b)the graph will show straight line with intercept
(c)the graph will show straight line with slope
(d)the graph will show straight line with intercept
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
For the reaction , the experimental data require the following rate equation:
Which of the following is/are true regarding this?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
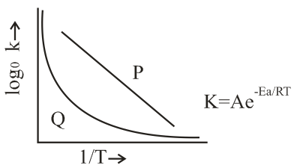
Which of the following statements are true regarding the plot shown in the given diagram?