EASY
Earn 100
For an elementary reaction if the volume of vessel is quickly increased to double of its original volume then the rate of reaction :
(a)Unchange
(b)Increases four times
(c)Increases two times
(d)Decreases eight times
50% studentsanswered this correctly
Important Questions on Chemical kinetics
EASY
The kinetic order for the following reaction is:
EASY
HARD
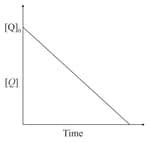
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
EASY
MEDIUM
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

EASY
MEDIUM
HARD
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
EASY
EASY
MEDIUM
MEDIUM
Which one of the following statements is correct?
EASY
EASY
EASY
EASY

