MEDIUM
NEET
IMPORTANT
Earn 100
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
(a)
(b)
(c)
(d)
77.78% studentsanswered this correctly

Important Questions on Electrochemistry
HARD
NEET
IMPORTANT
Consider the change in oxidation state of Bromine corresponding to different emf values, as shown in the diagram below:
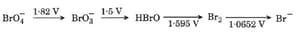
Then, what is the species undergoing disproportionation?
HARD
NEET
IMPORTANT
In the electrochemical cell:
the emf of this Daniel cell is . When the concentration is changed to and that of changed to the emf changes to . From the following, which one is the relationship between and ? (Given, )
MEDIUM
NEET
IMPORTANT
The molar conductivity of a solution of with electrolytic conductivity of at
EASY
NEET
IMPORTANT
During the electrolysis of molten sodium chloride, the time required to produce of chlorine gas using a current of is
EASY
NEET
IMPORTANT
If the for a given reaction has a negative value, which of the following gives the correct relationship for the values of and ?
EASY
NEET
IMPORTANT
The number of electrons delivered at the cathode, during electrolysis by a current of is (charge on electron )
MEDIUM
NEET
IMPORTANT
Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
HARD
NEET
IMPORTANT
The pressure of required to make the potential of hydrogen electrode zero in pure water at 298 K is:
