For the first order reaction the half life is . The time taken for completion of the reaction is mm. (Nearest integer)
Given :

Important Questions on Chemical Kinetics
A first order reaction has the rate constant, . The number of correct statement/s from the following is/are Given: .
A. Reaction completes in .
B. The reaction has a half-life of .
C. The time required for completion is 25 times the time required for 90% completion.
D. The degree of dissociation is equal to .
E. The rate and the rate constant have the same unit.
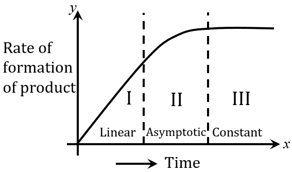
For certain chemical reaction , the rate of formation of product is plotted against the time as shown in the figure. The number of Correct statement/s from the following is _____
(A) Over all order of this reaction is one
(B) Order of this reaction can't be determined
(C) In region-I and III, the reaction is of first and zero order respectively
(D) In region-II, the reaction is of first order
(E) In region-II, the order of reaction is in the range of to .
An organic compound undergoes first order decomposition. If the time taken for the decomposition is , then the time required for decomposition will be is _____ s. (Nearest integer).
Given :
The rate constants of the above reaction at and are and respectively. The activation energy for the reaction is (Nearest integer)
(Given : In