For the first-order reaction (average life), and in the increasing order are:

Important Questions on Chemical Kinetics
In a hypothetical reaction, (first-order decomposition), is optically active (dextro-rotatory) while and are optically inactive, but takes part in a titration reaction (fast reaction) with . Hence, the progress of the reaction can be monitored by measuring the rotation of plane polarised light or by measuring volume of consumed in titration.
In an experiment, the optical rotation was found to be from start of the reaction. If the progress had been monitored by titration method, volume of consumed at (from start) is , then volume of consumed at will be
For the following parallel chain reaction, the overall half-life of is hours if the rate of formation of is of the rate of decomposition of , then what will be the half-life of while it is converting into ?
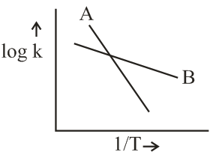
The Arrhenius relationship of two different reactions is shown below. Which reaction is faster of a lower temperature and which is more sensitive to changes of temperature?
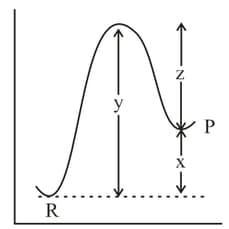
The potential energy diagram for the reaction is given below: of the reaction corresponds to the energy
Half-life period for decomposition of over tungsten wire are given below:
| Initial pressure in minutes | |||
Calculate the order of the reaction.
