For the following bond cleavage, use curved-arrows to show the electron flow and classify it as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion.
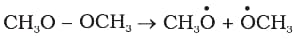

Important Points to Remember in Chapter -1 - Organic Chemistry – Some Basic Principles and Techniques from NCERT Chemistry Part II Textbook for Class XI Solutions
1. Organic chemistry:
(i) Organic chemistry is the study of hydrocarbon and their derivatives. Carbon in its compounds forms covalent bonds. The nature of the covalent bonding can be described in terms of orbitals hybridization concept. Carbon can undergo and hybridization in its compounds.
(ii) In a homologous series, any two successive members differ in their molecular mass by . and in their molecular formula by .
2. Nomenclature of organic compounds:
(i) IUPAC name of a compound is constituted from:
(ii) Secondary prefix Primary prefix Word root Primary suffix Secondary suffix.
(iii) The decreasing order of preferences of the secondary suffixes (functional groups) is :
Carboxylic acid sulphonic acid anhydride ester acid chloride acid amide nitrile aldehyde ketone alcohol amine.
3. Classification of organic compounds:
(i) Aliphatic (Acyclic) compounds are open chain compounds which contain straight or branched chain of carbon atoms.
(ii) Benzenoids are the aromatic compounds which contain one or more benzene ring.
4. Isomerism:
(i) Compounds having the same molecular formula but different physical and chemical properties are called isomers and the phenomenon is called isomerism.
(ii) Isomerism is broadly divided into two types. i. Constitutional isomerism, ii. stereo isomerism.
(iii) Chain or nuclear or skeletal isomerism: Isomers have similar molecular formula but differ in the nature of the carbon skeleton.
(iv) Position isomerism: If different compounds belonging to same homologous series with the same molecular formula and carbon skeleton, but differ in the position of substituent or functional group or an unsaturated linkage are said to exhibit position isomerism.
(v) Functional isomerism: Different compounds having same molecular formula, but different functional groups are said to exhibit functional isomerism.
(vi) Metamerism: This type of isomerism is a special kind of structural isomerism arises due to the unequal distribution of carbon atoms on either side of the functional group or different alkyl groups attached to the either side of the same functional group and having same molecular formula.
(vii) Tautomerism: It is a special type of functional isomerism in which a single compound exists in two readily interconvertible structures that differ markedly in the relative position of at least one atomic nucleus, generally hydrogen. The two different structures are known as tautomers.
(viii) The isomers which have same bond connectivity but different arrangement of groups or atoms in space are known as stereoisomers.
(ix) Geometrical isomers are the stereoisomers which have different arrangement of groups or atoms around a rigid framework of double bonds. This type of isomerism occurs due to restricted rotation of double bonds, or about single bonds in cyclic compounds.
(x) Compounds having same physical and chemical property but differ only in the rotation of plane of the polarised light are known as optical isomers and the phenomenon is known as optical isomerism.
5. Inductive effect:
(i) –I Effects of certain groups follow the order :
(ii) + I Effects of certain alkyl groups follow the order :
(iii) Inductive effect is a permanent effect while electromeric effect is temporary in nature.
6. Hyperconjugation:
Hyperconjugation is noticed in those species which have at least one α-hydrogen atom.
7. Resonance:
(i) The resonance structures (canonical structures or contributing structures) are hypothetical and individually do not represent any real molecule.
(ii) The difference in energy between the actual structure and the lowest energy resonance structure is called the resonance stabilisation energy or simply
the resonance energy.
(iii) The resonance effect is defined as ‘the polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and lone pair of electrons present on an adjacent atom.
(iv) There are two types of resonance or mesomeric effect designated as effect and effect.
8. Electromeric Effect (E effect):
It is a temporary effect. The organic compounds having a multiple bond (a double or triple bond) show this effect in the presence of an attacking reagent only.
9. Reaction intermediates:
(i) Carbocation is planar hybridised). The relative order of the stabilities of carbocations is:
(ii) Carbanion is pyramidal ( hybridised). The relative order of the stabilities of carbanions is :
(iii) Free radical is nearly planar hybridised). The relative order of stabilities of free radicals is :
(iv) Electrophiles are positively charged or electron deficient species.
(v) Nucleophiles are negatively charged or electron rich species.
10. Methods of purification of organic compounds:
(i) Crystallisation is used to purify organic solids by dissolving them in suitable solvents.
(ii) Fractional Crystallisation is used to separate two or more organic solids with different solubilities in the same solvent.
Camphor, benzoic acid, naphthalene etc. can be purified by sublimation process.
(iii) Simple distillation is used to purify liquids with non–volatile impurities or liquid mixture in which the components differ in boiling points by atleast
(iv) Fractional distillation is used to purify a liquid mixture in which the components differ in boiling points by to .
(v) Steam distillation can be used to purify organic compounds which give sufficient vapours at the boiling point of water and are not miscible with water.
(vi) Chromatography is particularly useful to purify and separate the constituents from a sample if available in a very small amount.
