MEDIUM
Earn 100
For the following reaction at the equilibrium:
If is added, then solubility of increases, because
(a) of increase due to addition of .
(b) of AgCl decrease due to addition of .
(c) react with and equilibrium shift in right side.
(d) react with and form so equilibrium shift in backward direction.
50% studentsanswered this correctly
Important Questions on Ionic Equilibrium
HARD
EASY
HARD
MEDIUM
HARD
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM
Which of the following choices is correct for a mixture of and
MEDIUM
MEDIUM
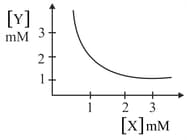
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
EASY
EASY
MEDIUM
HARD
MEDIUM

