MEDIUM
JEE Main
IMPORTANT
Earn 100
For the following reactions
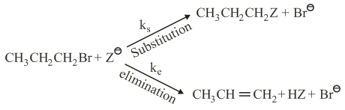
where,
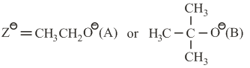
and , are respectively, the rate constants for substitution and elimination, and , the correct option is ________
(a) and
(b) and
(c) and
(d) and
33.33% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
For the reaction the observed rate expression is, rate . The rate expression for the reverse reaction is:
MEDIUM
JEE Main
IMPORTANT
During the nuclear explosion, one of the products is with half life of years. If of was absorbed in the bones of a newly born baby in place of how much time, in years, is required to reduce it by if it is not lost metabolically ____________.
EASY
JEE Main
IMPORTANT
The rate of a reaction doubles when its temperature changes from . Activation energy of such a reaction will be:
MEDIUM
JEE Main
IMPORTANT
For the non-stoichiometry reaction, , the following kinetic data were obtained in three separate experiments, all at .
| Initial Concentration (A) | Initial Concentration (B) | Initial rate of formation of C |
|---|---|---|
The rate law for the formation of is
EASY
JEE Main
IMPORTANT
The rate of a reaction decreased by times when the temperature was changed from . the activation energy (in ) of the reaction is ________ .
EASY
JEE Main
IMPORTANT
Consider the following reactions
The order of the above reactions are respectively. The following graph is obtained when log[rate] vs.log[conc.] are plotted:
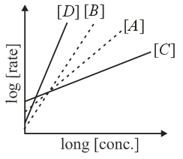
Among the following, the correct sequence for the order of the reactions is :
HARD
JEE Main
IMPORTANT
The rate constant of a reaction is measured at different temperature , and the data are plotted in the given figure. the activation energy of the reaction in is : is gas constant)
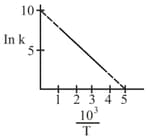
MEDIUM
JEE Main
IMPORTANT
A flask contains a mixture of compounds and . Both compounds decompose by first-order kinetics. The half-lives for and are and , respectively. If the concentrations of and are equal initially, the time required for the concentration of A to be four times that of (in s) is :
