HARD
JEE Main
IMPORTANT
Earn 100
For the following reactions
It was found that the is decreased by in the presence of catalyst. If the rate remains unchanged, the activation energy for catalysed reaction is (Assume pre-exponential factor is same)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
For the reaction, products, when the concentration of and both were doubled, the rate of the reaction increased from to When the concentration of alone is doubled, the rate increased from to
Which one of the following statements is correct?
MEDIUM
JEE Main
IMPORTANT
The following results were obtained during kinetic studies of the reaction.
product
| Experiment | Initial rate of reaction | ||
The time (in minutes) required to consume half of is
MEDIUM
JEE Main
IMPORTANT
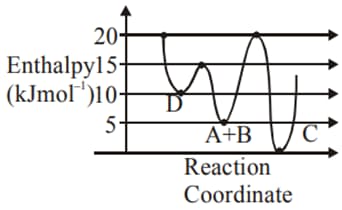
Consider the given plot of enthalpy of the following reaction between and
Identify the incorrect statement.
MEDIUM
JEE Main
IMPORTANT
The given plots represent the variation of the concentration of a reactant with time for two different reactions . The respective orders of the reaction are
(i) 
(ii) 
MEDIUM
JEE Main
IMPORTANT
The rate of a reaction quadruples when the temperature changes from to . The activation energy of this reaction is:
(Assume Activation energy and pre-exponential factor are independent of temperature; )
HARD
JEE Main
IMPORTANT
The reaction of ozone with oxygen atoms in the presence of chlorine atoms can occur by a two step process shown below:
...(i)
...(ii)
The closest rate constant for the overall reaction
is:
HARD
JEE Main
IMPORTANT
The half-life period of a first-order reaction is . The amount of substance left after one hour will be
MEDIUM
JEE Main
IMPORTANT
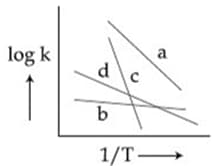
Consider the following plots of rate constant versus for four different reactions. Which of the following orders is correct for the activation energies of these reactions?