HARD
JEE Advanced
IMPORTANT
Earn 100
For the given close packed structure of a salt made of cation and anion shown below (ions of only one face are shown for clarity), the packing fraction is approximately (packing fraction )
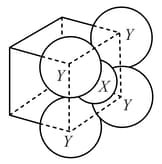

(a)
(b)
(c)
(d)
28.57% studentsanswered this correctly

Important Questions on Solid State
HARD
JEE Advanced
IMPORTANT
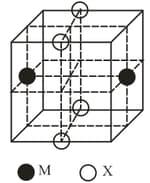
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
HARD
JEE Advanced
IMPORTANT
Consider an ionic solid , with structure. Construct a new structure , whose unit cell is constructed from the unit cell of , following the sequential instructions given below. Neglect the charge balance.
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
HARD
JEE Advanced
IMPORTANT
If the unit cell of a mineral has cubic close packed (ccp) array of oxygen atoms with m fraction of octahedral holes occupied by aluminium ions and n fraction of tetrahedral holes occupied by magnesium ions, m and n, respectively, are
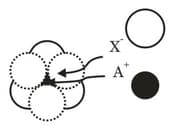
MEDIUM
JEE Advanced
IMPORTANT
The arrangement of X- ions around A+ ion in solid AX is given in the figure (not drawn to scale). If the radius of X- is 250 pm, the radius of A+ is