For the reaction, the plot of v/s is given below:
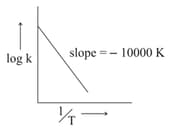
The temperature at which the rate constant of the reaction is is _____
(Rounded-off to the nearest integer) [Given : The rate constant of the reaction is at .]

(Rounded-off to the nearest integer) [Given : The rate constant of the reaction is at .]

Important Questions on Chemical Kinetics
Sucrose hydrolyses in acid solution into glucose and fructose following first order rate law with a half-life of at After the fraction of sucrose remaining is The value of is ________ (Rounded off to the nearest integer)
[Assume: In]
In the above first order reaction the initial concentration of is at The concentration of after hour was The rate constant of the reaction at is (Nearest integer):
Given
In the above first order reaction the concentration of reduces from initial concentration to in minutes at The rate constant for the reaction at is The value of is __________.
[Given ]
The results given in the below table were obtained during kinetic studies of the following reaction:
| Experiment | Initial rate/ | ||
| I | |||
| II | |||
| III | |||
| IV | X | ||
| V | Y |
X and Y in the given table are respectively :
