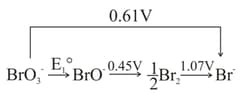For the reaction at equilibrium, the concentration of A is B is 0.15 M and C is 0.05 M. The for the hydrolysis of A at 300 K is kJ/mole. The value of X is?
Report your answer by rounding it upto nearest integer.
Important Questions on Electrochemistry
The Gibbs energy change (in ) for the given reaction at and is :
;
Take
at is approximately:
For the disproportionation reaction at (where is the equilibrium constant) is _______
Given
An oxidation-reduction reaction in which 3 electrons are transferred has a of at The value of (in V) is .
Relation between Gibb's Free Energy and EMF of a Cell Consider a efficient hydrogen-oxygen fuel cell working under standard conditions at bar and . Its cell reaction is
The work derived from the cell on the consumption of of is used to compress of a monoatomic ideal gas in a thermally insulated container. What is the change in the temperature (in ) of the ideal gas?
The standard reduction potentials for the two half-cells are given below.
,
Use .
is
The emf of the following cell is at .
The standard free energy change for the cell reaction is________
For the cell reaction
at The standard Gibbs energy of the cell reaction is:
[Given that Faraday constant ]
Given that:
The standard electrode potential and its temperature coefficient for a cell are and at , respectively. The reaction is . The standard reaction enthalpy at in is
Use and