EASY
NEET
IMPORTANT
Earn 100
For the reaction . If active mass of is kept constant and active mass of is tripled, the rate of forward reaction will become
(a)Three times
(b)Six times
(c)Eight times
(d)Nine times
50% studentsanswered this correctly

Important Questions on Equilibrium
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
For the reaction equilibrium : if at equilibrium and is total pressure, the ratio is equal to
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
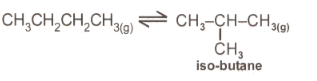
If the value of is , the percentage by mass of iso-butane in the equilibrium mixture would be
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
