MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
For the sublimation of a solid at , which of the following may be correct:
(a) at low temperature
(b)
(c) at high temperature
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Select the correct statements:
For every chemical reaction at equilibrium, the standard Gibbs energy of reaction is zero.
At constant temperature and pressure, chemical reactions are spontaneous in the direction of decreasing Gibbs energy.
Spontaneity is related to change in entropy of universe.
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
Look at the following diagram:
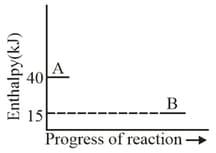
The enthalpy change for the reaction will be
MEDIUM
JEE Main/Advance
IMPORTANT
In Haber's process of manufacturing of ammonia:
| Molecule | |||
If is independent of temperature, then reaction at as compared to that of will be:
