EASY
JEE Main
IMPORTANT
Earn 100
For which combination of temperatures, the efficiency of Carnot's engine is highest?
(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
MEDIUM
JEE Main
IMPORTANT
Two carnot engines and are operated in succession. The first one, receives heat from a source at and rejects to the sinks at . The second engine receives heat rejected by the first engine and then rejects to another sink at . If the work outputs of the two engines are equal, then the value of is
MEDIUM
JEE Main
IMPORTANT
A carnot's engine whose low temperature reservoir is at has an efficiency of . It is desired to increase the efficiency to . By how many degrees should the temperature of the high temperature reservoir be increased?
MEDIUM
JEE Main
IMPORTANT
Six moles of an ideal gas performs a cycle shown in the figure. If the temperatures are , and then work done per cycle is approximately___ (Take )
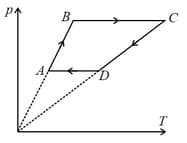
MEDIUM
JEE Main
IMPORTANT
A Carnot's engine is made to work between and first and then between and . The ratio of efficiencies of the engine in the two cases is
EASY
JEE Main
IMPORTANT
The process , for an ideal gas can be best represented in the form of a graph
EASY
JEE Main
IMPORTANT
When an air bubble rises from the bottom to the surface of a lake, its radius becomes double. Find the depth of the lake. Given that the atmospheric pressure is equal to the pressure due to a column of water high. Assume constant temperature and disregard surface tension
MEDIUM
JEE Main
IMPORTANT
Two identical containers joined by a small pipe initially contain the same gas at pressure and absolute temperature . One container is now maintained at the same temperature while the other is heated to . The common pressure of the gases will be
MEDIUM
JEE Main
IMPORTANT
An ideal monoatomic gas is compressed (no heat being added or removed in the process) so that its volume is halved. The ratio of the new pressure to the original pressure is
