HARD
Earn 100
For which of the following parameters the structural isomers and would be expected to have the same values? (Assume ideal behaviour)
(a)Heat of vaporisation
(b)Vapour pressure at the same temperature
(c)Boiling points
(d)Gaseous densities at the same temperature and pressure
20% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
MEDIUM
MEDIUM
MEDIUM
MEDIUM
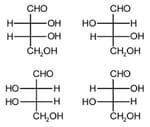
MEDIUM
MEDIUM
Which of the following alkenes can generate optically active compounds upon hydrogenation?
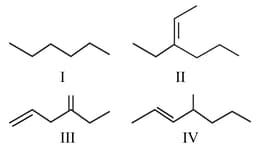
MEDIUM
EASY
HARD
What is isomerism? Write the names and structural formulas of the isomers of pentane.
MEDIUM
The structure of two organic compounds are given below:
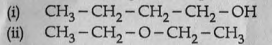
(a) Write the molecular formula of these compounds.
(b) Which type of isomerism do they exhibit?
(c) Explain the isomerism.
(d) Write the position isomer of butanol.
EASY
MEDIUM
In the following reaction sequence, identify the relationship between the products Q and S :
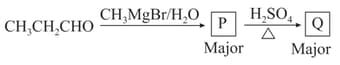
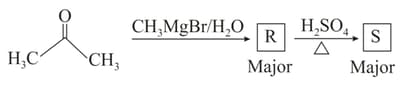
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM

