EASY
AP EAPCET
IMPORTANT
Earn 100
For zero order reaction, a plot of versus will be
(a)A straight line passing through the origin and slope
(b)A horizontal line (parallel to - axis)
(c)A straight line with slope
(d)A straight line passing through origin and slope
28.57% studentsanswered this correctly

Important Questions on Chemical Kinetics
EASY
AP EAPCET
IMPORTANT
Which statement among the following is incorrect?
EASY
AP EAPCET
IMPORTANT
For a products the rate of the reaction is given by Rate . The units of rate constant will be
MEDIUM
AP EAPCET
IMPORTANT
The rate constants for a reaction at and are and ,respectively. The activation energy of the reaction in is ______
EASY
AP EAPCET
IMPORTANT
The rate constant is same for reactions of order , and , respectively, the unit of concentration being in moles per litre. If the concentration of the reactant is unity, the rates of reaction will be
EASY
AP EAPCET
IMPORTANT
The rate equation for a first-order reaction is given by . A straight line with positive slope is obtained by plotting ( Initial concentration of the reactant, concentration of the reactant at time )
MEDIUM
AP EAPCET
IMPORTANT
If the rate constant for a first order reaction is , find the time required to reduce of the reactant to .
EASY
AP EAPCET
IMPORTANT
For an elementary reaction, , the is minutes. In what period of time would the concentration of be reduced to of its original concentration?
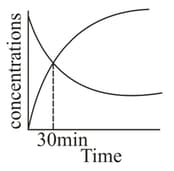
MEDIUM
AP EAPCET
IMPORTANT
For a first order reaction , the concentrations vs time plot is as shown. The half-life of the reaction is