EASY
Earn 100
Formal charges do not indicate real charge separation within the molecule.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
Which of the following compounds contain(s) no covalent bond(s)?
EASY
The numbers of lone pair and bond pairs in hydrazine are, respectively:
HARD
The lattice energies of and follow the order
MEDIUM
Give a reason for the following:
Ionic compounds have a high melting point.
MEDIUM
In the given electron dot structure, the formal charge on each nitrogen atom (respectively) from left to right is _______
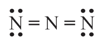
EASY
What do you understand by a lone pair of electrons?
MEDIUM
Among the following molecules, the one with the largest bond angle at the central atom is
EASY
Which of the following pair contains lone pair of electrons on the central atom?
MEDIUM
The formal charges of atoms and in the ion is
MEDIUM
The compound(s) with two lone pairs of electrons on the central atom is (are)
EASY
Arrange the following compounds in increasing order of bond length: methanol, phenol, -ethoxyphenol
MEDIUM
In ozone molecule, the formal charge on the central oxygen atom is
MEDIUM
The compound having longest bond is:
EASY
Total number of lone pair of electrons in ion is:
EASY
The number of -bonds and -bonds present in naphthalene are respectively
EASY
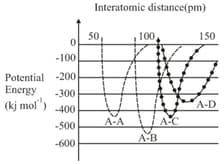
The intermolecular potential energy for the molecules , , and given below suggests that:
MEDIUM
The correct order of bond length is:
MEDIUM
Maximum bond angle at nitrogen is present in which of the following?
MEDIUM
The formal charge on central oxygen atom in ozone is
EASY
Which compound has both covalent as well as co-ordinate bonds?

