MEDIUM
JEE Advanced
IMPORTANT
Earn 100
Four particles have velocities and . The root mean square velocity of the particles (definition wise) is,
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermometry, Thermal Expansion and Kinetic Theory of Gases
MEDIUM
JEE Advanced
IMPORTANT
The temperature of an ideal gas is increased from to . The RMS speed of its molecules become,
HARD
JEE Advanced
IMPORTANT
In case of hydrogen and oxygen at , which of the following is the same for both?
EASY
JEE Advanced
IMPORTANT
The average kinetic energy of the molecules of an ideal gas at has the value . The temperature at which the kinetic energy of the same gas becomes is,
MEDIUM
JEE Advanced
IMPORTANT
A polyatomic gas with degrees of freedom has a mean energy per molecule given by,
EASY
JEE Advanced
IMPORTANT
In a process, the pressure of a gas remains constant. If the temperature is doubled, then the change in the volume will be,
MEDIUM
JEE Advanced
IMPORTANT
A steel rod of length is heated from to keeping its length constant. The longitudinal strain developed in the rod is (given, the coefficient of linear expansion of steel)?
MEDIUM
JEE Advanced
IMPORTANT
The coefficient of linear expansion of steel and brass are and , respectively. If their difference in lengths at all temperatures has to kept constant at , their lengths at should be respectively,
MEDIUM
JEE Advanced
IMPORTANT
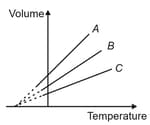
The expansion of an ideal gas of mass at a constant pressure is given by the straight line . Then, the expansion of the same ideal gas of mass at a pressure is given by the straight line