MEDIUM
NEET
IMPORTANT
Earn 100
From the following bond energies:
bond energy:
bond energy:
bond energy:
bond energy:
Enthalpy for the reaction,
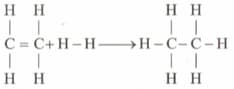
Will be:
bond energy:
bond energy:
bond energy:
bond energy:
Enthalpy for the reaction,

(a)
(b)
(c)
(d)
60.71% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
The following two reactions are known: and. The value of for the following reaction is
HARD
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Consider the following processes:
For will be:
MEDIUM
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
