MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
From the following data, determine the enthalpy change for the sublimation of ice at . [Mean heat capacity of ice, mean heat capacity of , mean heat capacity of , enthalpy of fusion of ice at , enthalpy of evaporation of water at ]

Important Questions on Thermodynamics & Thermochemistry
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The heat of formation of is . The heat of combustion of is for and are and respectively. Then determine the for the isomerisation reaction , and for the same are at .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
In the reaction, of , the enthalpies of formation of are in the ratio of and have opposite sign. Determine the value of .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
and are diatomic molecules. If the bond enthalpies of are in the ratio and enthalpy of formation of from and is . What is the bond enthalpy of ?
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
When a certain amount of ethylene was combusted, heat was evolved. If heat of combustion of ethylene is, determine the volume of (at ) that entered into the reaction.
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Given the following reactions:
I:
II:
Which amongst and is more stable?
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Enthalpy of polymerisation of ethylene, as represented by the reaction, is per mole of ethylene. Given bond enthalpy of bond is , determine enthalpy of bond (in ).
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Substance can undergo decomposition to form two sets of products:
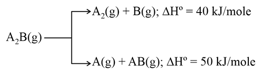
If the molar ratio of to is in a set of product gases, determine then the energy involved in the decomposition of mole of .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
For the hypothetical reaction:
If and are and , respectively at is then determine at .
